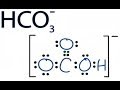




















The conjugate base of HCO3- is CO32-. Conjugates always differ by one H+. A conjugate base has one fewer H+, while a conjugate acid has one more H+. Finding conjugate acid and conj. base of HCO3-. In parts (c) and (d) of problem 11.1 from the textbook, we are asked to write the formula of the conjugate base of HCO3- (book answer: H2CO3) and the conjugate acid of HCO3- (book answer: CO3^2-). The conjugate base of HCO 3-is CO 3-2, which is the carbonate ion.. To determine the conjugate base of a substance, you remove one hydrogen ion. It's... See full answer below. Most of the conjugate acids you'll ever need to know : HCO3- : HCO3− Hydrogencarbonate (bicarbonate) ion. CO32− Carbonate ion ===== HFSbF5 Fluoroantimonic acid SbF6− Hexafluoroantimonate ion. HCl... So, in order to determine the conjugate acid for a given base, all you have to know is that "base" + H^(+) -> "conjugate acid of that base" In your case, the base is hydrogen carbonate, or HCO_3^(-). If you write the equation you'll get HCO_3^(-) + H^(+) -> H_2CO_3 Carbonic acid, or H_2CO_3, will be the conjugate acid of hydrogen carbonate. Answer to Part B What is the conjugate base of HCO3 ? Express your answer as a chemical formula. View Available Hint(s) - AED O ? thus the conjugate base of any compound is the compound after the removal of H+ from them respectively The conjugate base of acid HCO3- is (CO3)^2- The conjugate base of H2O is OH- Create your account. What is the conflict of the story of sinigang? Conjugates always differ by one H+. All other trademarks and copyrights are the property of their respective owners. H2CO3 is the acid (releases H+) ---> (HCO3)-1 is its conjugate base (will take that H+ back) H2O is the base (takes H+ ion) What is the conjugate base of HCO3-? Explanation. What is the conjugate base of HCO3-? Answer. The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-. HCO3- is a conjugate acid, H 2 CO 3. 1) What is the conjugate base of HCO3−? Express your answer as a chemical formula. 2) What is the conjugate acid of HPO32− ? Express your answer as a chemical formula. 3) Among three bases, X−, Y−, and Z−, the strongest one is Y−, and the weakest one is Z−. Rank their conjugate acids, HX, HY, and HZ, in order of decreasing strength.
[index] [714] [6815] [1124] [7959] [1690] [1689] [6803] [6500] [646] [391]
In this video we will learn the difference between strong and weak acids and bases. Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. It explains the concept, compo... A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl... If you want to understand acid-base analysis, then you need to understand buffers. In this short video, you will learn everything you need to know. A step-by-step explanation of how to draw the HNO3 Lewis Structure (Nitric Acid). The HNO3 Lewis structure is best thought of as the NO3 with an H attache... Pyruvic acid is placed in water at physiological pH (7.3). Under these conditions, which species will dominate; the conjugate base or the conjugate acid? The... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com
Copyright © 2024 m.topcasinoonline.shop